Software de mantenimiento de las ciencias de la vida para el cumplimiento de la normativa
Simplifique el cumplimiento y la calibración con la GMAO eMaint para las ciencias de la vida
Los gobiernos regulan estrictamente las empresas de ciencias de la vida. Los profesionales de mantenimiento y operaciones deben cumplir las leyes locales, estatales, federales e internacionales. Con tantas regulaciones, no es de extrañar que muchas organizaciones encuentren las normas de información y auditoría confusas y engorrosas. Ahí es donde entra en juego el software de sistemas informatizados de gestión del mantenimiento (CMMS ).
Como una de las soluciones de ciencias de la vida mejor valoradas, el GMAO eMaint simplifica el cumplimiento de la normativa
al crear un rastro de papel digital para los inspectores
Con la documentación digital, puede compartir fácilmente las pruebas de cumplimiento con los auditores. Las organizaciones de ciencias de la vida
no solo demostrarán el cumplimiento más fácilmente con registros y firmas digitales, sino que experimentarán una mayor fiabilidad operativa.
Las 5 principales ventajas de la GMAO para las ciencias de la vida
Creación de un registro de auditoría
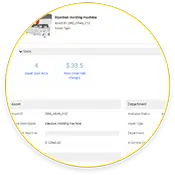
Los equipos de mantenimiento que utilizan eMaint CMMS crean pistas de cumplimiento de extremo a extremo para las auditorías de la Parte 11 del CFR, incluyendo historiales de activos, finalización de órdenes de trabajo y firmas electrónicas.
Racionalización de la salud y el historial de los activos
eMaint CMMS no sólo almacena los historiales de las órdenes de trabajo de los activos, sino que también incluye datos históricos y en tiempo real. monitoreo de condición en tiempo real.
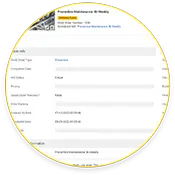
Automatización de la calibración y los MP
Las acciones de mantenimiento preventivo de conformidad y calibración pueden programarse o automatizarse mediante el GMAO eMaint.
Seguimiento de la mano de obra y el inventario
A medida que se completan las órdenes de trabajo, el seguimiento automático de la mano de obra y los niveles de inventario ayuda a los gerentes a planificar y programar futuros MP y pedidos de reabastecimiento.
Tomar decisiones financieras más inteligentes
Con los registros adecuados, el liderazgo puede tomar decisiones más informadas sobre la reparación/sustitución de activos, las implementaciones de IIoT y el mantenimiento centrado en la fiabilidad.

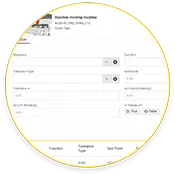
Calibración
Programar órdenes de trabajo de calibración para verificar la precisión de las mediciones de monitoreo de condición .
Simplifique las auditorías de cumplimiento automatizando las órdenes de trabajo de calibración y los calendarios de seguimiento de las órdenes de trabajo de mantenimiento preventivo (PM).
Aumente la precisión agilizando el mantenimiento de registros con actividades digitalizadas, como la calibración, las pruebas y las mediciones.
Reduzca los dolores de cabeza de las auditorías de cumplimiento eliminando las voluminosas carpetas y reduciendo los errores de introducción de datos en los registros.
Activos que necesitan ser calibrados
Centrífugas
Elementos de presión
Indicadores de presión
Escalas
Temperatura
Presión
Métricas de flujo
Pipetas
Incubadoras
Verticales de ciencias de la vida que se benefician de una GMAO
Agricultura, Pesca y Alimentación
Anatomía y morfología
Ciencias del comportamiento
Biología
Bioquímica
Biotecnología
Biofísica
Botánica
Citología
Ecología y medio ambiente
Entomología
Silvicultura
Genética y herencia
Inmunología
Microbiología
Micología
Parasitología
Productos farmacéuticos
Farmacología
Fisiología
Ciencias vegetales
Toxicología
Ciencias Veterinarias
Virología
Zoología
Y más