Life Sciences Maintenance Software for Regulatory Compliance
Simplify compliance and calibration with eMaint CMMS for life sciences
Governments strictly regulate life science businesses. Maintenance and operations professionals must comply with local, state, federal, and international laws. With so many regulations, it’s no wonder many organizations find the reporting and auditing standards confusing and cumbersome. That’s where computerized maintenance management systems (CMMS) software comes in.
With digital documentation, you can easily share proof of compliance with auditors. Not only will life sciences organizations
prove compliance easier with digital records and signatures, but they will experience increased operational reliability.
Top 5 CMMS Benefits for Life Sciences
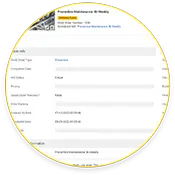
Creating Audit Trail
Maintenance teams using eMaint CMMS create end-to-end compliance trails for 21 CFR Part 11 audits, including asset histories, work order completions, and electronic signatures.
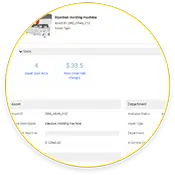
Streamlining Asset Health and History
Not only does eMaint CMMS store asset work order histories, but it also includes real-time and historical condition monitoring data.
Automating Calibration & PMs
Compliance and calibration preventive maintenance actions can be scheduled or automated using eMaint CMMS.
Tracking Labor and Inventory
As work orders are completed, automatically tracking labor and inventory levels helps managers plan and schedule future PMs and restock orders.
Making Smarter Financial Decisions
With proper records, leadership can make more informed decisions about asset repair/replacements, IIoT implementations, and reliability-centered maintenance.

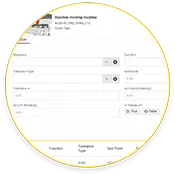
Calibration
Schedule calibration work orders to verify the accuracy of condition monitoring measurements.
Simplify compliance audits by automating calibration work orders and tracking schedules for preventive maintenance (PM) work orders.
Increase accuracy by streamlining record-keeping with digitalized activities, including calibration, tests, and measurements.
Reduce compliance audit headaches by eliminating bulky binders and reducing data entry errors in records.
Assets That Need Calibration
Centrifuges
Pressure Elements
Pressure Indicators
Scales
Temperature
Pressure
Flow Metrics
Pipettes
Incubators
Life Sciences Verticals That Benefit From a CMMS
Agriculture, Fisheries, & Food
Anatomy & Morphology
Behavioral Sciences
Biology
Biochemistry
Biotechnology
Biophysics
Botany
Cytology
Ecology & Environment
Entomology
Forestry
Genetics & Heredity
Immunology
Microbiology
Mycology
Parasitology
Pharmaceuticals
Pharmacology
Physiology
Plant Sciences
Toxicology
Veterinary Sciences
Virology
Zoology
And more